Quality Improvement Initiatives
As a Leading Company in the Silicone Industry
- HOME
- Quality Improvement Initiatives
Safe and Stable Product Supply System
with a High-Level Quality Management System
Since the foundation, we have manufactured our products and made efforts to improve quality
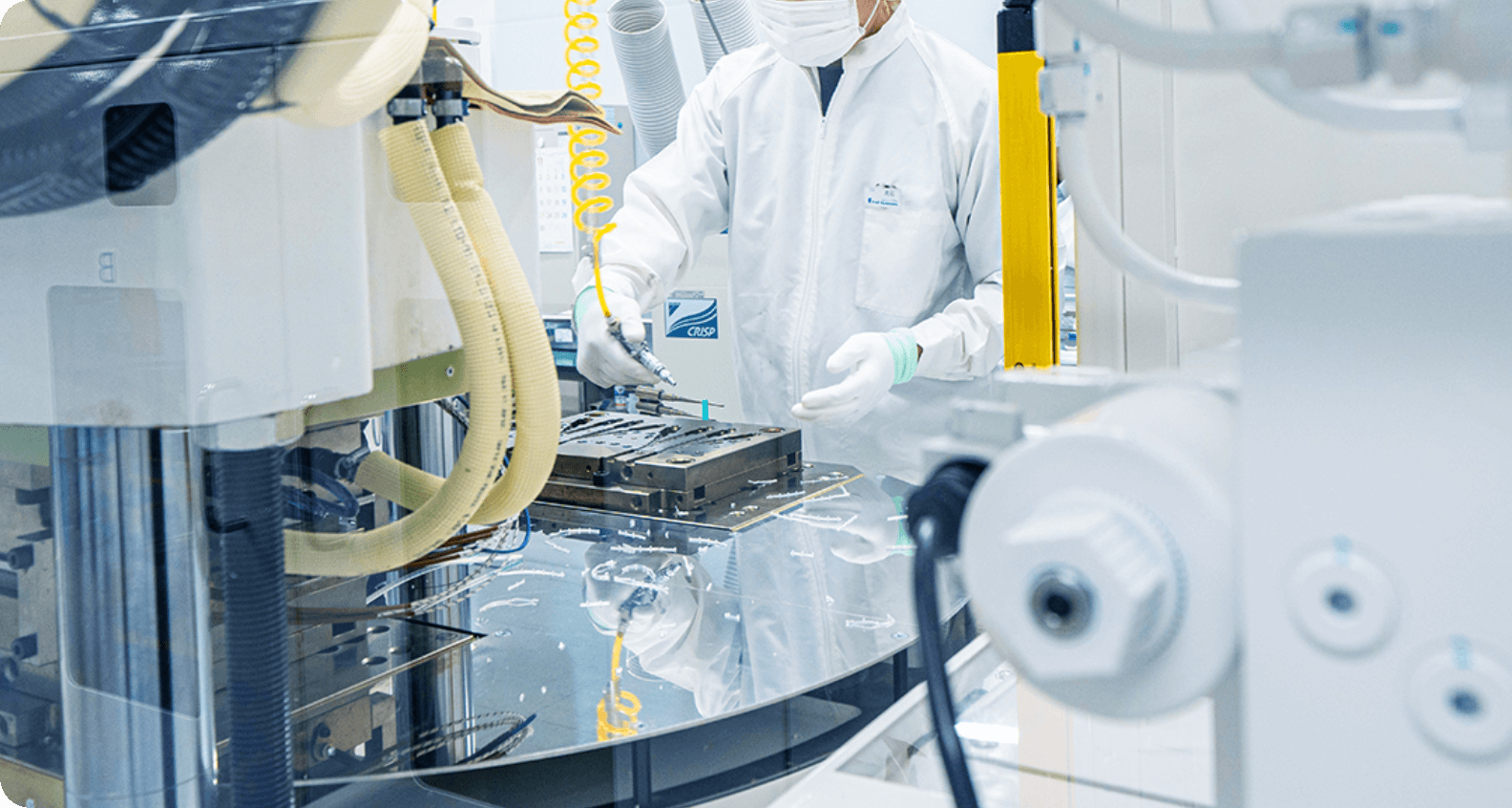
in compliance with the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices and the QMS Ordinance as a general manufacturer of silicone medical devices based on our policy of "For the benefit of patients.” On the manufacturing floor, we implement strict product control in all processes from raw materials to final products.

Certification in International Standards Including ISO 13485
As a manufacturer of medical devices, we improve our product supply system based on various international standards and quality management systems of different countries and regions. We comply with laws, regulations, and codes including the Medical Device QMS Standard (ISO 13485), EU Medical Device Regulation (MDR), and Medical Device Single Audit Program (MDSAP). We also produce medical devices based on our own strict control system that we have been developing over the years.
Timely Improvement for Quality Improvement
Production of medical devices requires safety and high reliability. We improve product structures and functions based on feedback from medical professionals. Furthermore, we strive to improve our production technology and process on an ongoing basis for a safer and more stable supply after thorough consideration in terms of quality assurance and Good Vigilance Practice (GVP).

Manufacturing and Inspection by Qualified Personnel
We have strict in-house standards for manufacturing and inspection. Only personnel who have passed the standards are allowed to perform manufacturing and inspection. We also perform validation and total inspection based on product risks.




